Aquariums are a popular way to house fish and other aquatic creatures. In order to keep your aquarium healthy, it is important to understand the concept of pH and Alkalinity.
pH or power of Hydrogen is a measure of how acidic or basic a liquid is, while Alkalinity measures the level of calcium hardness of the water. In this blog post, we will explore the difference between pH and Alkalinity, and discuss how each affects your aquarium. We will also provide tips for maintaining a healthy aquarium environment.
pH and Alkalinity in Marine Aquarium
1. pH in Aquarium
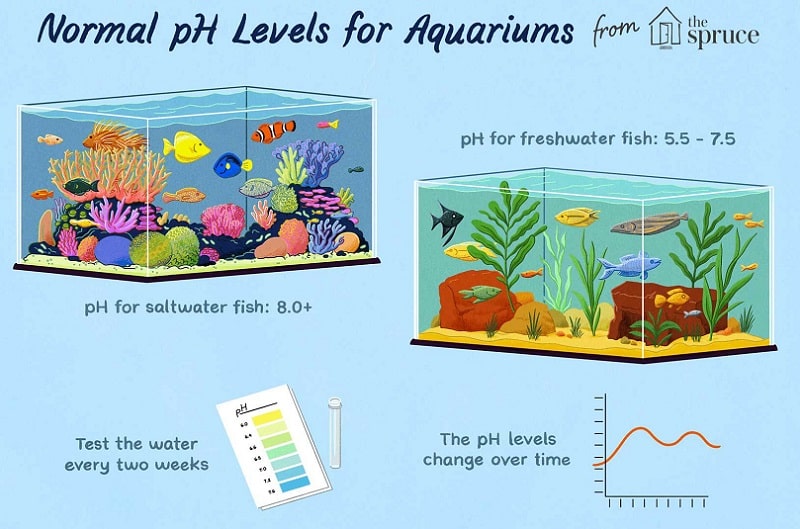
pH is a measure of the hydrogen ion concentration in water. The pH scale ranges from 0 to 14, with 7 being neutral.
A pH less than 7 is considered acidic, while a pH greater than 7 is considered basic or alkaline. Therefore, the lower the pH, the more acidic the water. The higher the pH, the more basic the water.
2. Alkalinity in Aquarium
Alkalinity is a measure of the ability of water to resist changes in pH. Water with high Alkalinity can buffer against changes in pH, keeping it relatively stable. Alkalinity is measured in calcium carbonate (CaCO3) equivalents.
Like pH, Alkalinity is also measured on a scale from 0 to 14. A value of 0 means that the water is very acidic, while a value of 14 indicatesindicates that the water is very basic.
3. The right level: Alkalinity and pH
Aquariums should have a pH between 6.5 and 8.0, with 7.0 being neutral. The ideal Alkalinity for an aquarium is between 2 and 3 meq/L.
Aquariums with low Alkalinity are more prone to sudden changes in pH, which can be harmful to fish and other aquatic creatures.
The relationship between alkalinity and pH levels is crucial to maintaining a healthy fish tank. Understanding the impact of each on the other can help you make informed decisions about your aquarium. Here are some resources that can help you gain a more comprehensive understanding of this relationship.
Relationship Between Alkalinity And pH
The terms alkalinity and pH are often used interchangeably in the aquarium hobby. Alkalinity and pH are closely related, but they are not the same.
Since Alkalinity is a measure of water’s buffering capacity, pH is a measure of the hydrogen ion concentration in water, Alkalinity affects pH, but pH does not affect Alkalinity.
Both are important for a healthy aquarium. By maintaining the proper levels of both, you can create a safe and welcoming environment for your fish and other aquatic creatures.
Alkalinity is usually expressed in terms of milligrams per liter (mg/L) of calcium carbonate (CaCO3), while pH is typically expressed on a scale from 0 to 14, with 7 being neutral.
A solution with high Alkalinity will have a higher capacity to resist changes in pH. In contrast, a solution with low Alkalinity will be more prone to fluctuations.

Generally speaking, higher Alkalinity is better for aquariums since it provides a more stable environment for plants and fish.
However, it’s important to keep in mind that each species has different requirements and some may do better in slightly acidic or basic conditions.
To maintain a healthy aquarium environment, it is important to test the water regularly and make adjustments as needed.
There are a variety of test kits available that can be used to test for pH and Alkalinity. Most pet stores also offer water testing services.
What should be adjusted first: Alkalinity or pH?
In general, it is best to adjust Alkalinity first, because this will have a direct effect on pH.
Alkalinity acts as a buffer, so by increasing Alkalinity you can raise the pH of the water. However, if pH is too low, it can be not easy to raise it without also raising Alkalinity.
There are a few exceptions to this rule. If your water has high levels of dissolved organic carbon (DOC), adjusting Alkalinity first may not have the desired effect on pH. This is because DOC can bind to Alkalinity and affect the way it affects pH. In these cases, it is best to adjust pH first and then Alkalinity.
It’s also important to keep in mind that each aquarium is different and that some trial and error may be necessary to find the perfect balance for your particular setup.

What is more important, pH or Alkalinity?
While both pH and Alkalinity play crucial roles in maintaining a healthy aquarium, Alkalinity is more important in some cases. It acts as a buffer, stabilizing pH levels and preventing rapid changes.
A stable pH is essential for the well-being of your aquatic friends.
How does Alkalinity affect pH in reef tanks?

Reef tanks are usually kept at a higher pH than non-reef aquariums because many reef-dwelling organisms require a higher pH for optimal growth.
The typical range for pH in reef tanks is 8.0-8.4.
Alkalinity is also important in reef aquariums because it helps to buffer the water and maintain a stable pH.
The ideal range for Alkalinity in reef tanks is 3-5 meq/L.
Changes in pH and Alkalinity can significantly impact the health of reef tank inhabitants.
For example, if the Alkalinity in a reef tank decreases, the pH will also decrease. This change can be harmful to reef-dwelling organisms because many require a specific range of pH for optimal growth.
Monitoring pH and Alkalinity is important for maintaining optimal water quality in reef aquariums. Regular testing can help to identify problems before they become too severe. We should correct small imbalances before they have a chance to cause major issues.
It is important to maintain a stable pH in reef tanks because many of the animals that live in reef tanks are sensitive to changes in pH. For example, some corals will only grow in waters with a specific pH range. If the pH of the water changes too much, the coral will die.
To maintain a stable pH, reef tank owners often add alkalinity-increasing chemicals, such as sodium bicarbonate, to their tank water. By doing this, they can raise the Alkalinity of the water and help to buffer against sudden changes in pH.
Should I lower pH or Alkalinity first?
If you need to make adjustments, it’s generally better to start with lowering Alkalinity. By doing so, you can also lower the pH to some extent.
However, each aquarium is unique, so you may need to experiment to find the right approach for your setup.
Does high Alkalinity mean low pH?
Not necessarily. While Alkalinity can influence pH levels, a high Alkalinity does not always mean a low pH.
Alkalinity’s primary role is to resist pH changes, ensuring a more stable environment for your fish and plants.
Is raising Alkalinity the same as raising pH?
Raising Alkalinity is not the same as raising pH. Increasing Alkalinity helps buffer against pH fluctuations, but it may not directly raise pH levels.
To adjust pH, you may need to use other methods or treatments.
What to do if pH is good but Alkalinity is high?
If your pH is within the desired range but Alkalinity is high, it’s essential to find a balance. Consider using methods to lower Alkalinity while monitoring pH levels closely.
Keep in mind that each aquarium’s needs may differ, so regular testing and adjustments are crucial.
Is Alkalinity the same as PH?
Alkalinity and pH are closely related and are often measured together in aquariums. pH is a measure of the concentration of hydrogen ions or the acidity of water. Alkalinity, on the other hand, is the ability of water to neutralize or buffer changes in acidity
Source: Orenda Technologies
Conclusion
Alkalinity and pH are two important water parameters to monitor in an aquarium. While they both measure different aspects of the water, they are closely related. And, indeed they should be kept within certain levels to ensure the health of the fish and plants in the tank.
By understanding what each parameter means and how to test for them, you can better care for your aquatic friends. Have you tested your aquarium’s Alkalinity and pH lately?
Reference source: https://waterquality.montana.edu/well-ed/interpreting_results/fs_alkalinity_ph_tds.html




